Current Research
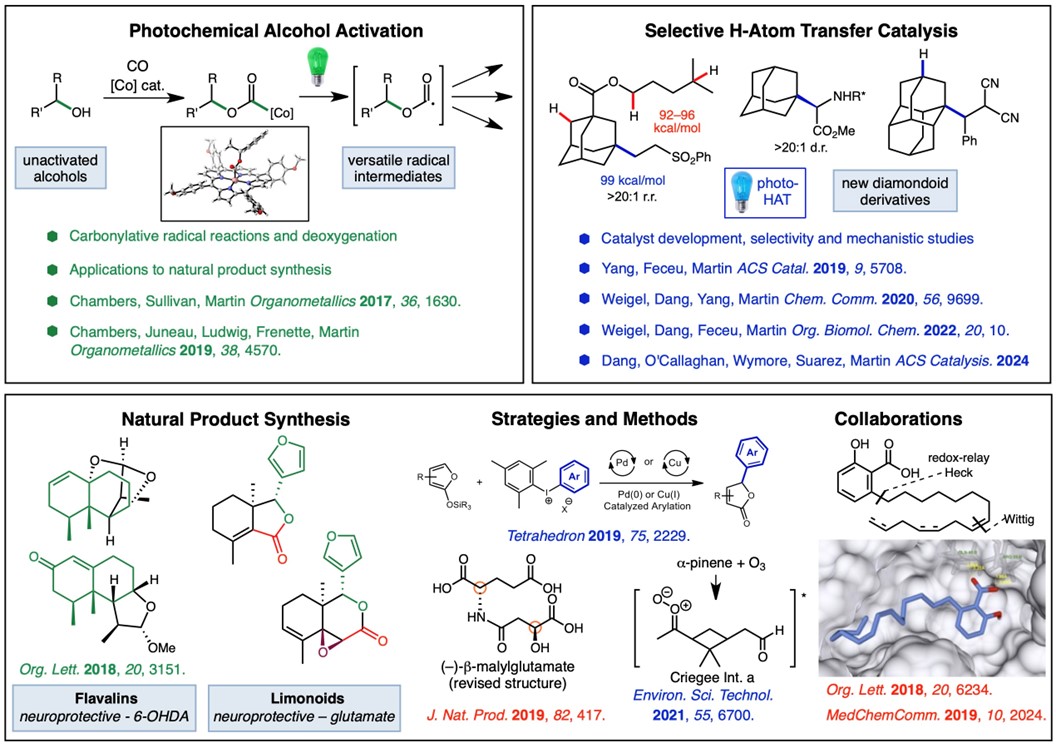
Sustainable Catalysis
We are also investigating the use of H-atom transfer (HAT) in activating C–H bonds for functionalization of a variety of hydrocarbons and simple molecules. We have a longstanding interest in the functionalization of diamondoids, rigid caged hydrocarbons that map onto the diamond lattice. We reviewed the direct radical functionalization (C-H to C-C via radicals) of diamondoids in OBC 2022. In 2019, we reported a visible light-mediated method for the direct alkylation of diamondoids such as adamantane and diamantane that proceeds with a unique selectivity profile (ACS Catalysis 2019). The direct substitution of various substituted adamantanes and drug derivatives occurs at the strongest C–H bond in the presence of a variety of common functional groups, including electronically activating N- and O-derivatives. This work was highlighted in 2019 (link) and was more recently expanded to aminoalkylation to access N-substituted derivatives with antiviral properties and the core of saxagliptin, a diabetes drug (ChemComm 2020). More recently, we explored a new mechanism for diamondoid functionalization using highly oxidizing pyrylium photocatalysts (ACS Catalysis 2024). This work featured extensive mechanistic studies (EPR, Stern-Volmer luminescence quencing, cyclic voltammetry) and the functionalization of higher diamondoids such as triamantane and tetramantane. Ongoing research aims to understand the underlying mechanistic principles and develop new strategies for site-selective C–H functionalization reactions. This research is supported by a MIRA grant from the NIH (R35-GM138050).
More recently, we have become interested in the use of iron catalysts for C-H functionalization reactions, with the goal of developing general reactions that use visible light rather than ultraviolet (UV) light. We have discovered a wavelength-selective catalytic reaction that provides new insight into the role of ligand-to-metal-charge transfer (LMCT) in iron catalysis.

Design and Synthesis of Bioactive Molecules
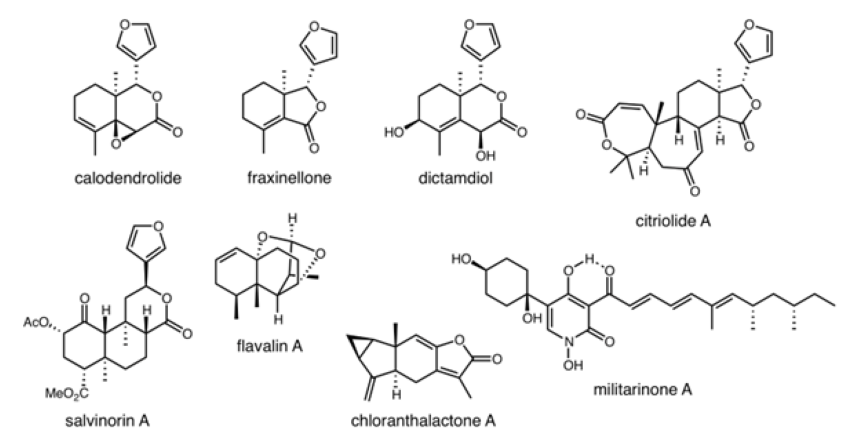
We are currently developing synthetic strategies to neuroprotective natural products, such as the limonoids, to provide probe molecules and investigate their mechanism of action. In collaboration with other researchers in the Iowa Neuroscience Institute (INI), including the Doorn Lab, we are testing natural products and small molecule analogs for neuroprotective activity. We have synthesized fraxinellone, calodendrolide, and many unnatural analogs, and have identified simplidied analogs with significantly improved neuroprotective activity against glutamate toxicity and environmental neurotoxins, such as pesticides. The ultimate goal is to provide molecular tools for the investigation of neurodegenerative processes and structure-activity relationship data for the development of neuroprotective small molecules as drugs for the treatment of neurodegenerative disease. This research is supported by a MIRA grant from the NIH (R35-GM138050).
Targets of Interest
Funding Sources

National Science Foundation CAREER Award – Catalysis CHE-1952860
(2018-2024)

National Institutes of Health MIRA Award R35-GM138050 (2020-Present)

University of Iowa
College of Liberal Arts and Sciences
(2019-Present)

Doctoral New Investigator Grant
Petroleum Research Fund
American Chemical Society
(2017–2019)

UC Riverside College of Natural and Agricultural Sciences
(2017-2018)

UC Riverside Center for Catalysis
seed grant
(2017-2018)

UC Cancer Research Coordinating Committee
(2018)